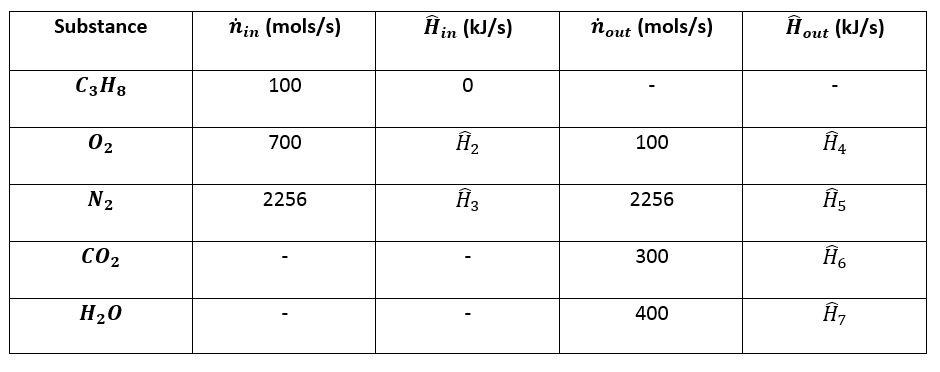
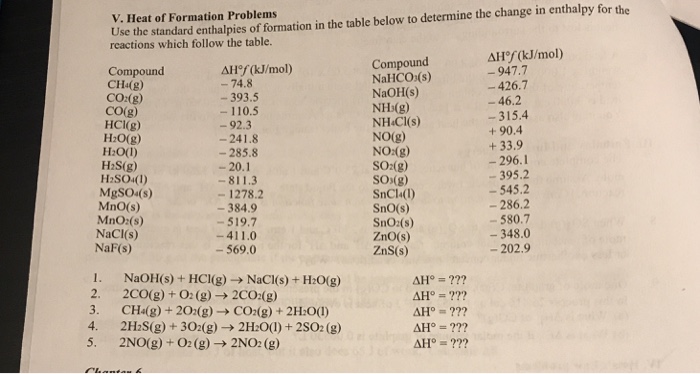
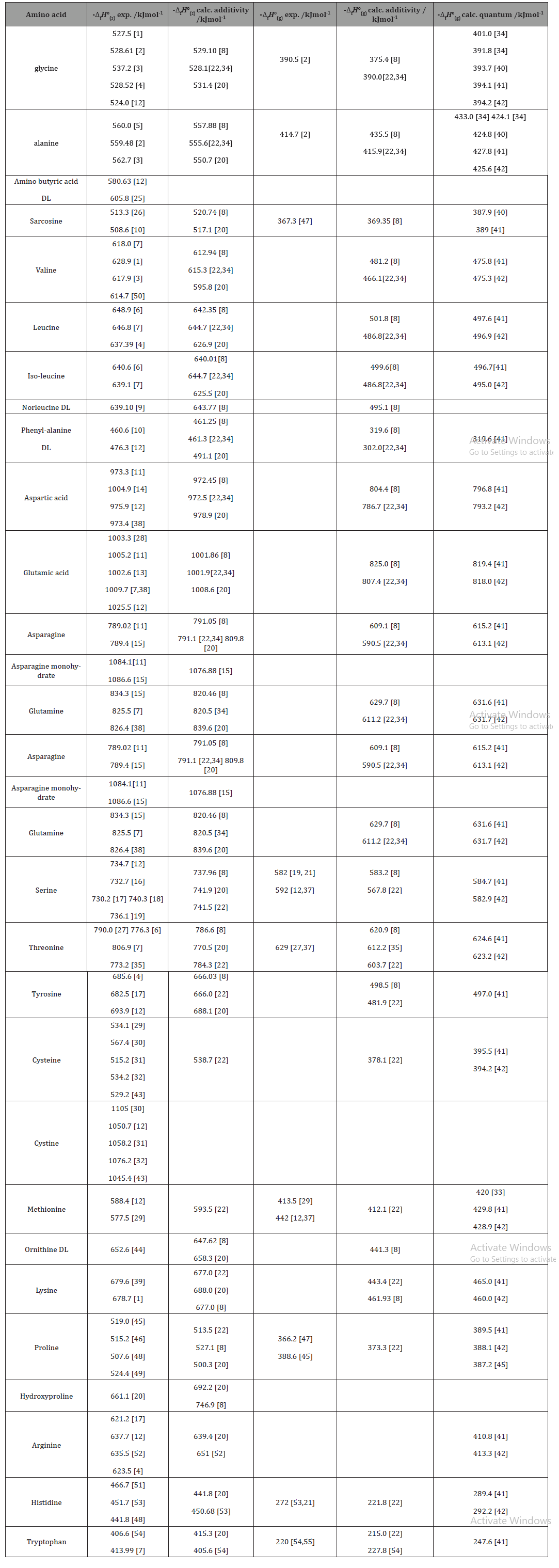
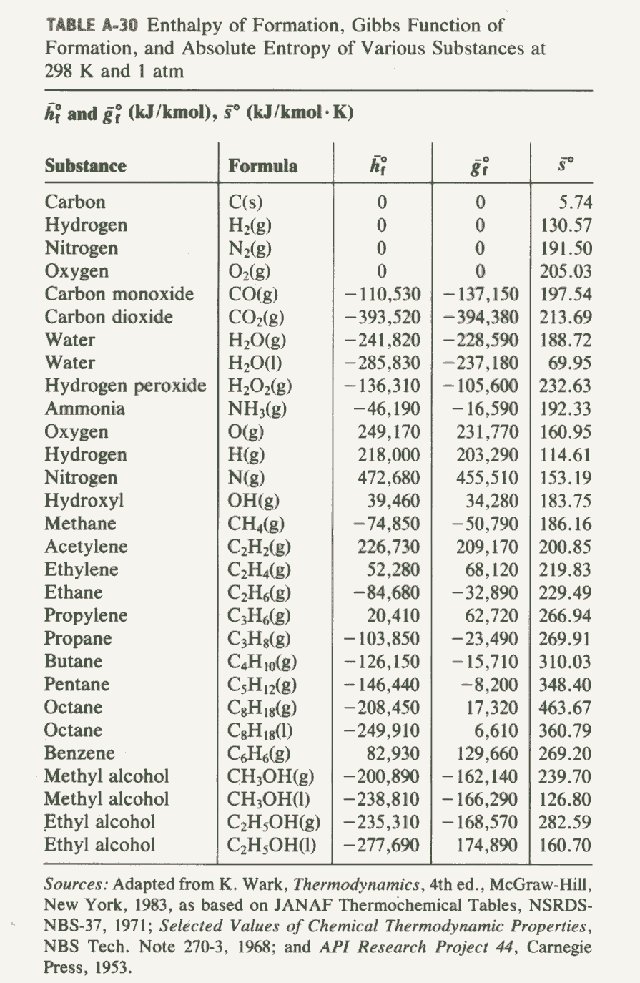
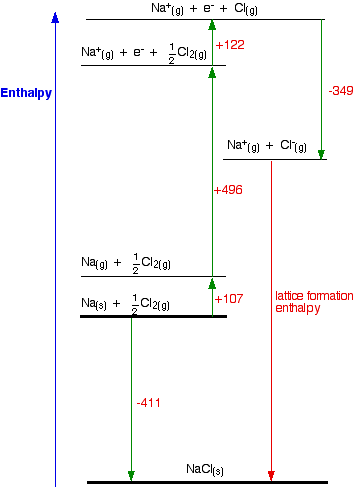
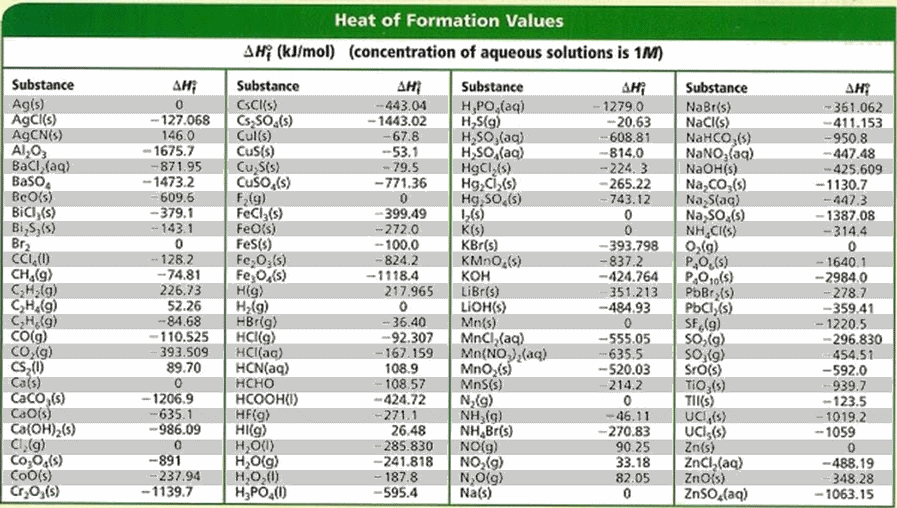
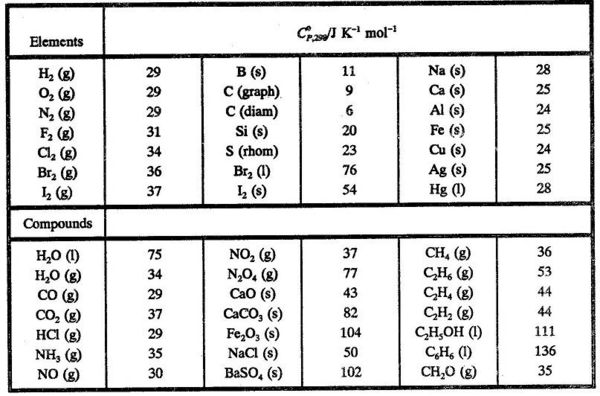
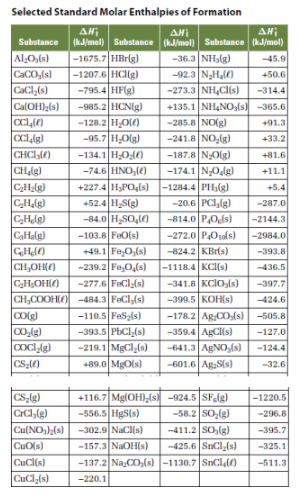
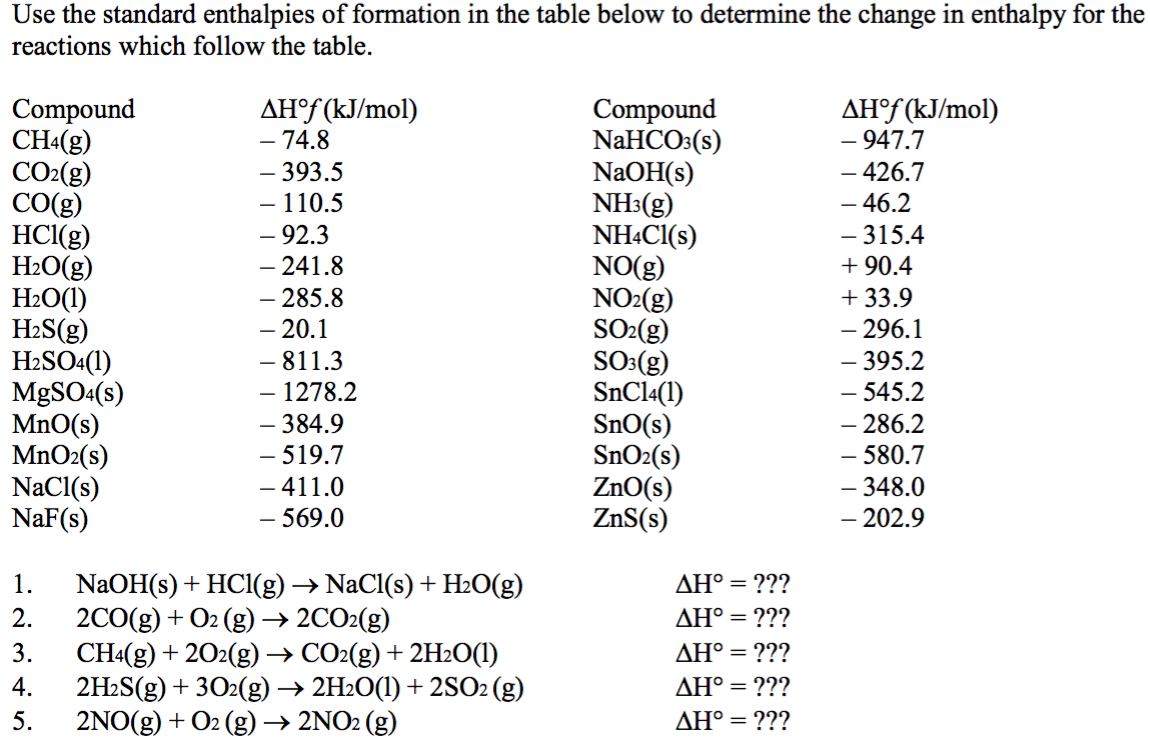
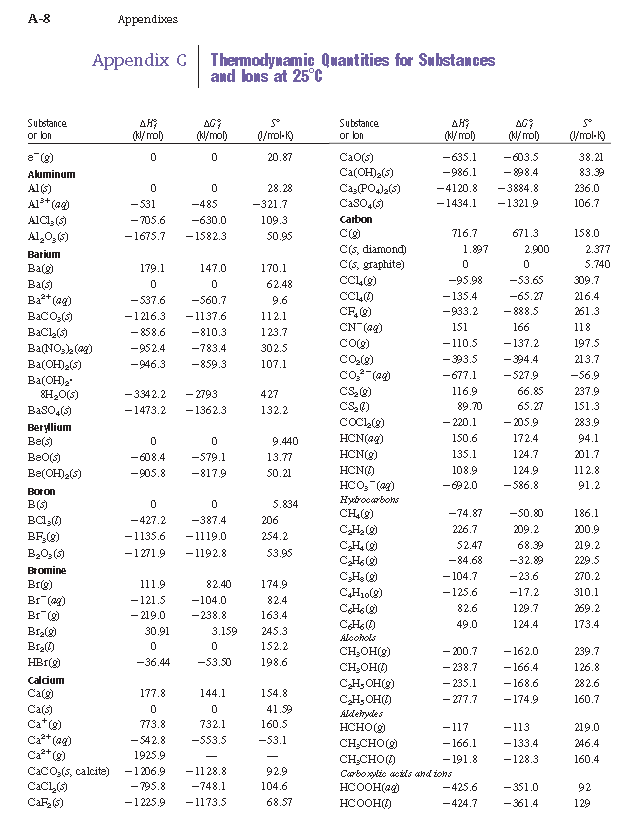
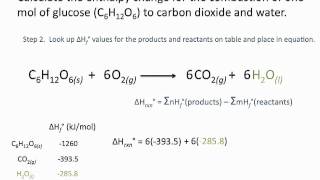Table 6 from Structure and heats of formation of iodine fluorides and the respective closed-shell ions from CCSD(T) electronic structure calculations and reliable prediction of the steric activity of the free-valence electron
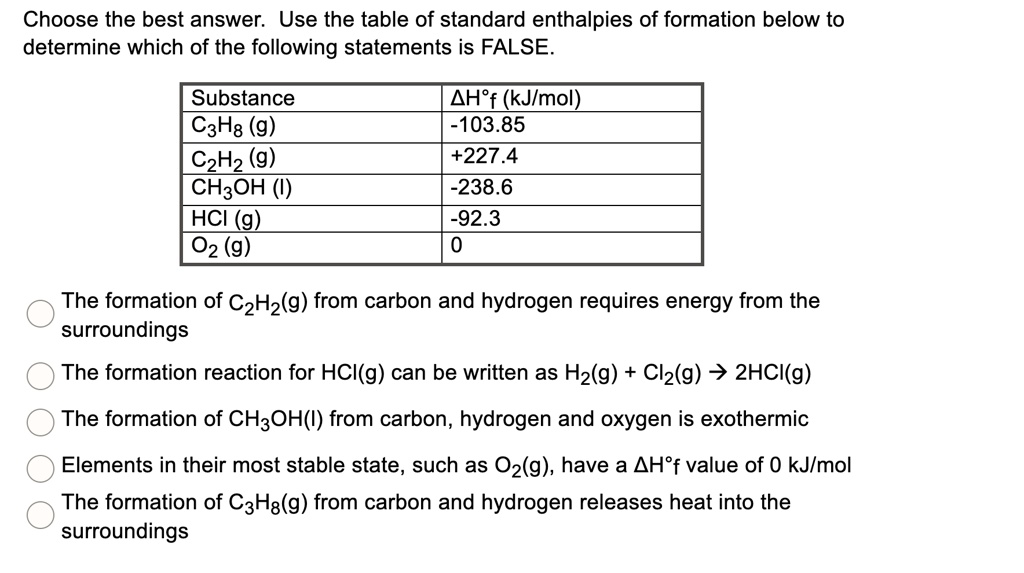
SOLVED: Choose the best answer: Use the table of standard enthalpies of formation below to determine which of the following statements is FALSE. Substance C2H2 (g) C2H4 (g) CH3OH HCl O2 (g)

Table 3 from Group additivity values for enthalpies of formation (298 K), entropies (298 K), and molar heat capacities (300 K < T < 1500 K) of gaseous fluorocarbons | Semantic Scholar
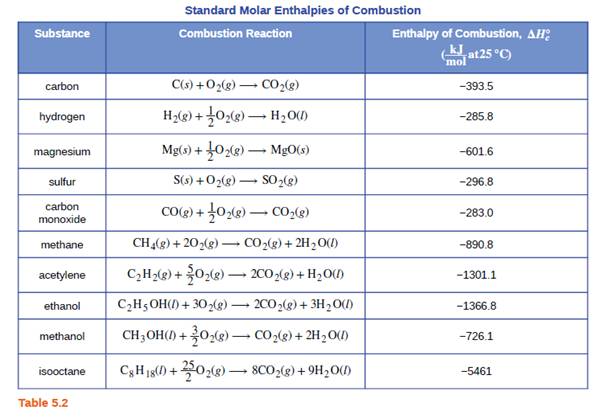
Which of the enthalpies of combustion in Table 5.2 the table are also standard enthalpies of formation? | bartleby

Solved) - The value for the standard heat of combustion, ?H° combustion, for... (1 Answer) | Transtutors

2009, Prentice-Hall, Inc. Enthalpies of Formation An enthalpy of formation, H f, is defined as the enthalpy change for the reaction in which a compound. - ppt download